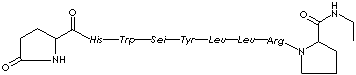|
LEUPROLIDE ACETATE |
| Synonyms. Leuprorelin acetate; des-Gly10-[D-Leu6]-LH-RH ethylamide; 5-Oxo-L-prolyl-L-histidyl-L-tryptophyl- L-seryl-L- tryosyl- D-leucyl- L- leucyl- L-arginyl-N-ethyl-L-prolinamide monoacetate; Leuprorelin acetate; Glp-His-Trp-Ser-Tyr-Leu-Leu-Arg-Pro-NHEt; Lucrin; Lupron; Procrin; Viadur; Eligard; Enantone; |
|
|
| PRODUCT IDENTIFICATION | |
|
CAS RN |
53714-56-0 (parent), 74381-53-6 (Acetate) |
|
EINECS RN |
256-342-8, 232-693-2 |
|
FORMULA |
C59H84N16O14 |
|
MOLE WEIGHT |
1241.40 |
|
H.S CODE |
2937.19.0000 |
|
SMILES |
c1cc2c(cc1)c(C[C@@H](C(=O)N[C@@H](C(=O)N[C@@H](Cc1ccc(cc1) O)C(N[C@@H](C(N[C@H](C(=O)N[C@H](C(N1CCC[C@H]1C(=O)NCC)= O)CCCNC(N)=N)C(C)C)=O)C(C)C)=O)CO)NC ([C@H](Cc1[nH]cnc1)NC([C @@H]1NC(CC1)=O)=O)=O) c[nH]2.OC(C)=O |
|
CLASSIFICATION |
Antineoplastic, Fertility Agent |
|
EXTRA NOTES |
A potent synthetic long-acting agonist of gonadotropin-releasing hormone that regulates the synthesis and release of pituitary gonadotropins, luteinizing hormone and follicle stimulating hormone |
|
|
| PHYSICAL AND CHEMICAL PROPERTIES | |
|
PHYSICAL STATE. |
white powder |
|
MELTING POINT |
|
|
BOILING POINT |
|
|
DENSITY |
|
|
SOLUBILITY IN WATER |
|
| SOLVENT SOLUBILITY | |
|
VAPOR DENSITY |
|
|
log P(octanol-water) |
|
|
VAPOR PRESSURE |
|
|
AUTOIGNITION TEMP |
|
| pH |
|
|
REFRACTIVE INDEX |
|
|
FLASH POINT |
|
|
|
| STABILITY AND REACTIVITY | |
| STABILITY | Stable under normal conditions. |
|
INCOMPATIBLE MATERIALS |
Strong acids, Strong bases |
| POLYMERIZATION |
Has not been reported |
|
NFPA RATINGS |
Health: 2,Flammability:0, Reactivity: 0 |
|
|
| EXTERNAL LINKS & GENERAL DESCRIPTION | |
|
Leuprolide: The active ingredient in a drug used to treat symptoms of advanced prostate cancer. It is also used to treat early puberty in children and certain gynecologic conditions. It is being studied in the treatment of other conditions and types of cancer. Leuprolide blocks the body from making testosterone (a male hormone) and estradiol (a female hormone). It may stop the growth of prostate cancer cells that need testosterone to grow. It is a type of gonadotropin-releasing hormone analog. (http://www.cancer.gov/) Leuprolide is FDA approved for the treatment of advanced prostate cancer. It is important for patients to remember that physicians have the ability to prescribe medication for conditions other than those for which the drug has been approved by the FDA. Patients who have received a prescription of this drug for a condition other than which it is approved may wish to discuss this issue with their physician. Leuprolide is classified as a luteinizing hormone releasing hormone (LHRH) agonist. LHRH is a naturally occurring hormone in the body that is involved in stimulating the production of female and male hormones such as estrogen and testosterone. Leuprolide inhibits the signaling of LHRH so that the production of estrogen or testosterone is reduced. Certain cancers, such as prostate cancer, are stimulated to grow from hormones. The reduction in the production of growth-stimulatory hormones inhibits the growth of cancer cells. (http://www.ufscc.ufl.edu/) Leuprolide is a luteinizing hormone releasing hormone (LHRH) agonist. It is a synthetic analog of LHRH (also known as gonadotropin releasing hormone [GnRH]).3 LHRH agonists (LHRHa) initially stimulate the release of luteinizing hormone (LH, gonadotropin), resulting in a transient elevation in serum androgen in men and serum estradiol in women. However, chronic administration can cause down-regulation of the LHRH receptors, thus inhibiting the secretion of LH and ultimately the sex hormones (androgen, estradiol). By decreasing the testicular production of androgen in men, LHRHa can inhibit the growth of androgen-dependent prostate cancer. Similarly, LHRHa reduce the ovarian secretion of estradiol and progesterone in women,4 leading to inhibition of estrogen-dependent cancers. In men, LHRHa can reduce serum androgen to castrate level. Similarly, in pre-menopausal women, serum estradiol level is reduced to post-menopausal levels. These decreases occur within two to four weeks after initiation of treatment, and are maintained as long as treatment continues. LHRHa are 50-100 times more potent than LHRH.5 In addition, they have a longer duration of action due to increased receptor affinity and greater biological stability. (http://www.bccancer.bc.ca/)
|
|
|
| SALES SPECIFICATION |
|
|
APPEARANCE |
white to off-white powder |
| MASS BALANCE | 97.0~103.0% |
|
PURITY |
98.0% min |
| AMINO ACID | 10% max (theoretical Composition) |
|
RELATED SUBSTANCES |
2.0% max (total impurity) |
| PEPTIDE CONTENT |
80.0%
min (N determination)
|
| ACETATE CONTENT |
12.0% max |
| WATER CONTENT | 6.0% max |
| BACTERIAL ENDOTOXINS | 5EU/mg max |
|
|
| TRANSPORT & REGULATORY INFORMATION | |
|
UN NO. |
Not known |
| HAZARD CLASS |
|
| PACKING GROUP | |
|
|
| SAFETY INFORMATION |
|
|
HAZARD OVERVIEW |
Reproductive hazard. Not a dangerous substance according to GHS |
| HAZARD CODES |
|
|
RISK PHRASES |
|
|
SAFETY PHRASES |
26 |
|
|
| PACKING |
|
|